产品编号:GM-87960MAB
产品名称:Anti-IGHE hIgG1 Reference Antibody(Omalbio)
目录价:询价

产品编号:GM-87960MAB
产品名称:Anti-IGHE hIgG1 Reference Antibody(Omalbio)
目录价:询价

GM-87985MAB-1mg / 1 mg
GM-87985MAB-5mg / 5 mg
GM-87985MAB-25mg / 5 mg * 5 vials
GM-87985MAB-50mg / 50 mg
GM-87985MAB-100mg / 50 mg * 2 vials
Expression System | CHO |
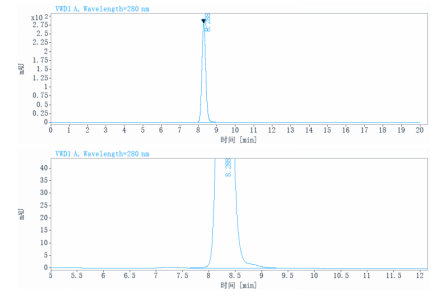
Aggregation | < 5% as determined by SEC-HPLC |
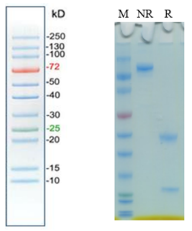
Purity | > 95% as determined by SDS-PAGE |
Endotoxin | < 1 EU/mg, determined by LAL gel clotting assay |
Sterility | 0.2 μm Filtered |
Target | IGHE |
Clone | omalizumab |
Alternative Names | IgE |
Source/Isotype | Human IgG1 (KEEM), Kappa |
Application | Bioactivity-ELISA |
Description | Omalizumab achieves its efficacy by targeting and neutralizing IgE (immunoglobulin E) . Ige plays an important role in allergic reactions, and Omalizumab reduces its amount, thereby reducing the occurrence of allergic symptoms. IgE-related research has driven the development of IgE-targeted biologics, and omalizumab, as the first anti-IgE agent, has demonstrated clear efficacy in moderate-to-severe asthma and chronic spontaneous urticaria, and has been used in the treatment of asthma and chronic urticaria, it provides a new option for patients who do not respond well to traditional treatments. |
Formulation | phosphate-buffered solution, pH 7.2-7.4. |
产品编号:GM-87960MAB
产品名称:Anti-IGHE hIgG1 Reference Antibody(Omalbio)
目录价:询价

GM-87985MAB-1mg / 1 mg
GM-87985MAB-5mg / 5 mg
GM-87985MAB-25mg / 5 mg * 5 vials
GM-87985MAB-50mg / 50 mg
GM-87985MAB-100mg / 50 mg * 2 vials
Expression System | CHO |
Aggregation | < 5% as determined by SEC-HPLC |
Purity | > 95% as determined by SDS-PAGE |
Endotoxin | < 1 EU/mg, determined by LAL gel clotting assay |
Sterility | 0.2 μm Filtered |
Target | IGHE |
Clone | omalizumab |
Alternative Names | IgE |
Source/Isotype | Human IgG1 (KEEM), Kappa |
Application | Bioactivity-ELISA |
Description | Omalizumab achieves its efficacy by targeting and neutralizing IgE (immunoglobulin E) . Ige plays an important role in allergic reactions, and Omalizumab reduces its amount, thereby reducing the occurrence of allergic symptoms. IgE-related research has driven the development of IgE-targeted biologics, and omalizumab, as the first anti-IgE agent, has demonstrated clear efficacy in moderate-to-severe asthma and chronic spontaneous urticaria, and has been used in the treatment of asthma and chronic urticaria, it provides a new option for patients who do not respond well to traditional treatments. |
Formulation | phosphate-buffered solution, pH 7.2-7.4. |

