产品编号:GM-87985MAB
产品名称:Anti-TNFSF13B(BAFF) hIgG1 Reference Antibody (Belibio)
目录价:询价

产品编号:GM-87985MAB
产品名称:Anti-TNFSF13B(BAFF) hIgG1 Reference Antibody (Belibio)
目录价:询价

GM-87985MAB-1mg / 1 mg
GM-87985MAB-5mg / 5 mg
GM-87985MAB-25mg / 5 mg * 5 vials
GM-87985MAB-50mg / 50 mg
GM-87985MAB-100mg / 50 mg * 2 vials
Expression System CHO
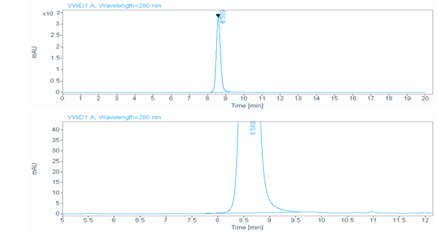
Aggregation < 5% as determined by SEC-HPLC
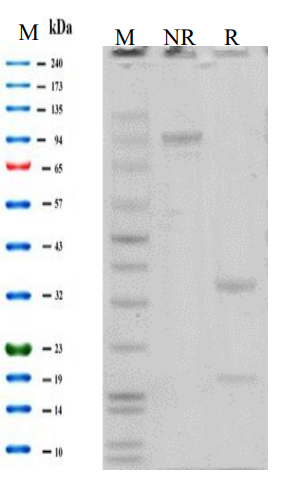
Purity >95% as determined by SDS-PAGE
Endotoxin <1 EU/mg, determined by LAL gel clotting assay
Sterility 0.2 μm Filtered
Target TNFSF13B(BAFF)
Clone Belimumab
Alternative Names BLYS, CD257, DTL, TALL-1, TALL1, THANK, TNFSF20, TNLG7A, ZTNF4
Source/Isotype Monoclonal Human IgG1(KDEL), Lambda2
Application /
Description BAFF (B-cell Activating Factor), also known as BLYS, CD257, TALL-1, THANK, and TNFSF20, is a member of the tumor necrosis factor superfamily, encoded by the TNFSF13B gene. Belimumab, a monoclonal antibody targeting BAFF, is marketed under the name Benlysta and is the first biologic globally approved for the treatment of systemic lupus erythematosus (SLE). Belimumab blocks the interaction of BAFF with its receptors (BAFF-R, TACI, and BCMA), thereby reducing B cell activation and the production of autoreactive antibodies.
Formulation phosphate-buffered solution, pH 7.2-7.4.

产品编号:GM-87985MAB
产品名称:Anti-TNFSF13B(BAFF) hIgG1 Reference Antibody (Belibio)
目录价:询价

GM-87985MAB-1mg / 1 mg
GM-87985MAB-5mg / 5 mg
GM-87985MAB-25mg / 5 mg * 5 vials
GM-87985MAB-50mg / 50 mg
GM-87985MAB-100mg / 50 mg * 2 vials
Expression System CHO
Aggregation < 5% as determined by SEC-HPLC
Purity >95% as determined by SDS-PAGE
Endotoxin <1 EU/mg, determined by LAL gel clotting assay
Sterility 0.2 μm Filtered
Target TNFSF13B(BAFF)
Clone Belimumab
Alternative Names BLYS, CD257, DTL, TALL-1, TALL1, THANK, TNFSF20, TNLG7A, ZTNF4
Source/Isotype Monoclonal Human IgG1(KDEL), Lambda2
Application /
Description BAFF (B-cell Activating Factor), also known as BLYS, CD257, TALL-1, THANK, and TNFSF20, is a member of the tumor necrosis factor superfamily, encoded by the TNFSF13B gene. Belimumab, a monoclonal antibody targeting BAFF, is marketed under the name Benlysta and is the first biologic globally approved for the treatment of systemic lupus erythematosus (SLE). Belimumab blocks the interaction of BAFF with its receptors (BAFF-R, TACI, and BCMA), thereby reducing B cell activation and the production of autoreactive antibodies.
Formulation phosphate-buffered solution, pH 7.2-7.4.


